Standard Operating Procedure Guidelines Fda
Standard operating procedures display of information education and communication materials relevant reference materials e g republic acts who gdp and gsp guide philippine national drug formulary standard practice guidelines pharmacovigilance related references the abovementioned additional documents will serve as proof of.
Standard operating procedure guidelines fda. Inadequate standard operating procedures sops are one of the most frequently cited causes of many deficiencies and observations found in forms 483 and warning letters and while specific sop issues can often be traced back to poor communication monitoring and or enforcement a poorly written sop can quietly grow into a host of other major compliance problems. Measures must be taken to ensure that the copies which are to be distributed are identical to the original standard operating procedure and distribution control must be maintained for approved copies or. Sop s must be available for every task that is used in the manufacture or testing of a regulated product. This standard operating procedures sop.
Sop s ensure that all processes and standard operation procedure are consistently replicated so even when there are. 8 26 2004 4 30 00 pm company. Standard operating procedures sops are issued to specifically instruct employees in areas of responsibility work instructions appropriate specifications and required records. The standard operating procedure or sometimes referred to as standard operating practices sop s are used to ensure that production processes are consistently and repeatedly executed exactly in accordance with a proven methodology.
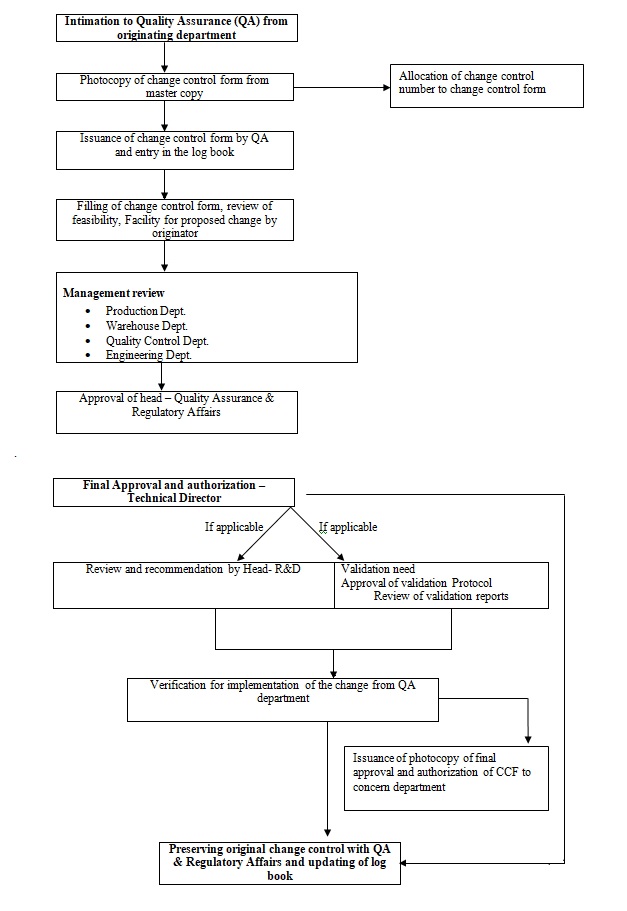
Operating procedures sops for pharmaceuticals good distribution practice good storage practices and other related activities with a view to integrate and standardize internal quality assurance systems of pharmaceutical importers and wholesalers in ethiopia. The sop gmp or sometimes referred to as standard operating practices sop s are used to lay out operations consistently and repeatedly in the same repetitive manner. Sops outline procedures which must be followed to claim compliance with gmp principles or other statutory rules and regulations. Standard operating procedures relevant to the activity must be available at the workplaces as well as the current version of the forms flow charts or other attachments to be used.
Sop s must be available for every task that is used in the manufacture or testing of a regulated product. Sop s are written step by step procedure that quality. Manual is provided with the latest essential information regarding the antifungal drug susceptibility testing methods such as broth micro dilution method and disk diffusion testing for yeasts and moulds including revised breakpoints.